Radioactivity is a natural phenomenon linked to the instability of certain atoms that make up matter.
Some radiation (X and gamma) is said to be ionizing because it emits rays of sufficient energies to transform the atoms they pass through into ions (an atom that has lost or gained one or more electrons). This can cause the material to become unstable.
An atom — unstable by nature or after contact with radiation — seeks to stabilize itself by emitting various types of radiation:
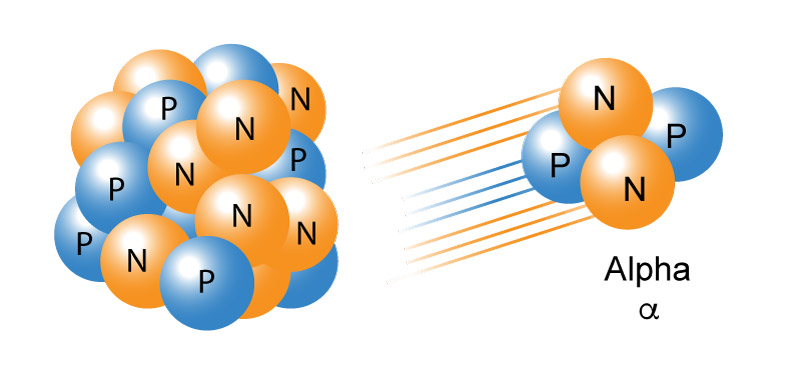
Alpha radiation occurs when an atom undergoes radioactive decay, giving off a particle (called an alpha particle) composed of two protons and two neutrons (essentially the nucleus of a helium-4 atom), changing the original atom into one of the elements with an atomic number 2 times lower and an atomic weight 4 times lower than its original state. Because of their charge and mass, alpha particles interact strongly with matter and only move a few centimeters in the air. Alpha particles are unable to penetrate the outer layer of dead skin cells, but are capable, if an alpha emitting substance is ingested in food or air, of causing serious cell damage. Polonium-210, for example, is an alpha emitter.
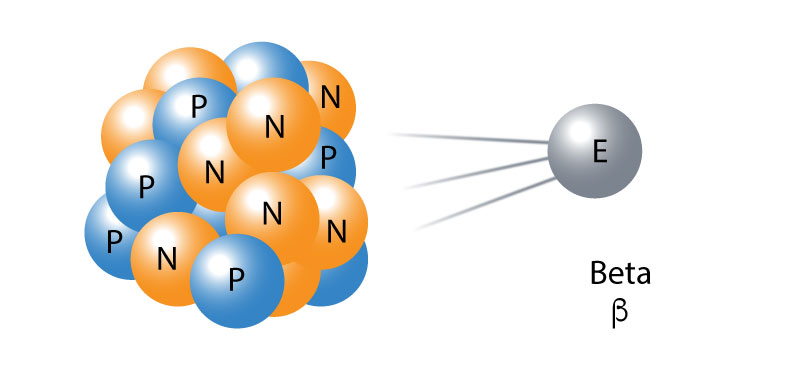
Beta radiation takes the form of an electron or a positron (a particle the size and mass of an electron, but with a positive charge) emitted from an atom. Because of its smaller mass, it is able to travel farther in the air, up to a few meters, and can be stopped by a thick piece of plastic or even a stack of paper. It can penetrate the skin by a few centimeters, posing a kind of risk to external health. However, the main threat lies primarily in the internal emission of the ingested material.
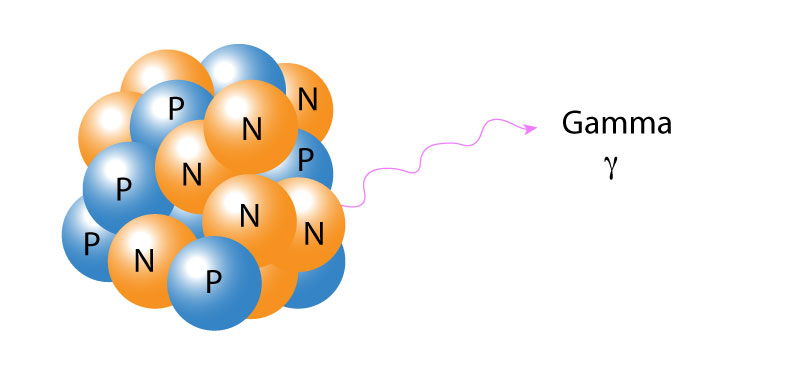
Gamma radiation, unlike alpha or beta, consists of no particles, and instead consists of an energy photon emitted from an unstable nucleus. Having no mass or charge, gamma radiation can travel much farther through the air than alpha or beta radiation, losing (on average) half of its energy every 152 meters. Gamma waves can be stopped by a sufficiently thick or dense layer material, with materials with a high atomic number such as lead, or depleted uranium being the most effective form of shielding.
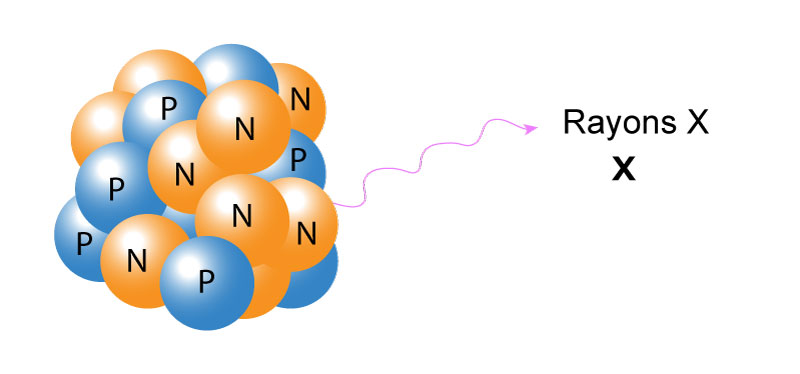
X-rays are similar to gamma radiation, with the primary difference being that they come from the electron cloud. This phenomenon is usually caused by energy changes in an electron, such as the transition from a higher energy level to a lower one, causing excess energy to be released. X-rays also have longer wavelengths and (generally) lower energy than gamma radiation.

Finally, neutron radiation consists of a free neutron, generally emitted as a result of spontaneous or induced nuclear fission. Capable of traveling hundreds or even thousands of meters in the air, they can however be effectively stopped if they are blocked by a material rich in hydrogen, such as concrete or water. Generally unable to ionize an atom directly due to their lack of charge, neutrons most often ionize indirectly, in the sense that they are absorbed into a stable atom, making the latter unstable and more likely to emit ionizing radiation of another type. In fact, neutrons are the only type of radiation that can make other materials radioactive.
Many sectors of activity are exposed to ionizing radiation, the main ones being:
All applications involving materials containing radioactive substances of natural origin (treatment of rare earths, production of phosphate fertilizers, oil production, refractory ceramics industry, etc.) are also concerned with the prevention of risks associated with ionizing radiation.
Radiation protection consists in assessing the risks associated with exposure to ionizing radiation and, if necessary, in implementing protective and preventive measures to reduce these risks.
With more than 50 years of experience in the nuclear industry, Baumert has designed, developed and qualified high-quality doors that limit the propagation of neutron and gamma rays and thus protect employees against excessive exposure to ionizing radiation. These doors are available in standard dimensions for free passage, but also for non-standard opening sizes, thanks to our custom design and manufacturing capabilities.
